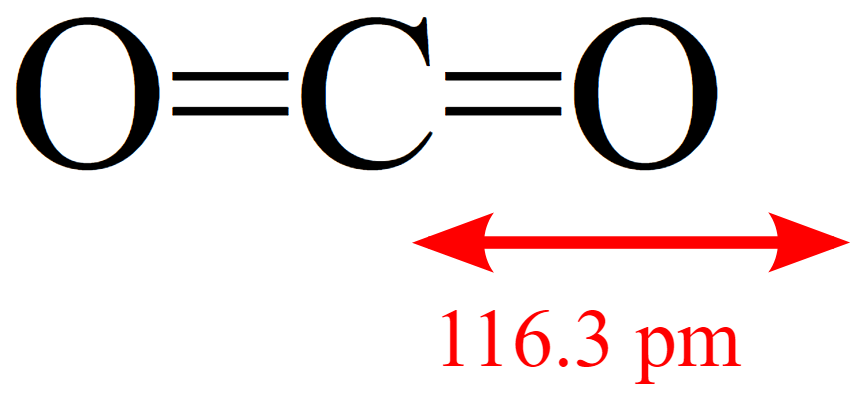Selectively Regulating Lewis Acid–Base Sites in Metal–Organic Frameworks for Achieving Turn‐On/Off of the Catalytic Activity in Different CO2 Reactions - Tian - 2022 - Angewandte Chemie International Edition - Wiley Online Library

Lewis Acid Strength of Interfacial Metal Sites Drives CH3OH Selectivity and Formation Rates on Cu‐Based CO2 Hydrogenation Catalysts - Noh - 2021 - Angewandte Chemie International Edition - Wiley Online Library
Synthesis of well-defined yttrium-based Lewis acids by capturing a reaction intermediate and catalytic application for cycloaddition of CO2 to epoxides under atmospheric pressure - Catalysis Science & Technology (RSC Publishing)

Fe(II) complexes: reservoirs for Lewis acids and carbenes and their utility in the conversion of CO2 to oxazolidinones - ScienceDirect

Lewis Acid Enhancement of Proton Induced CO2 Cleavage: Bond Weakening and Ligand Residence Time Effects | Journal of the American Chemical Society

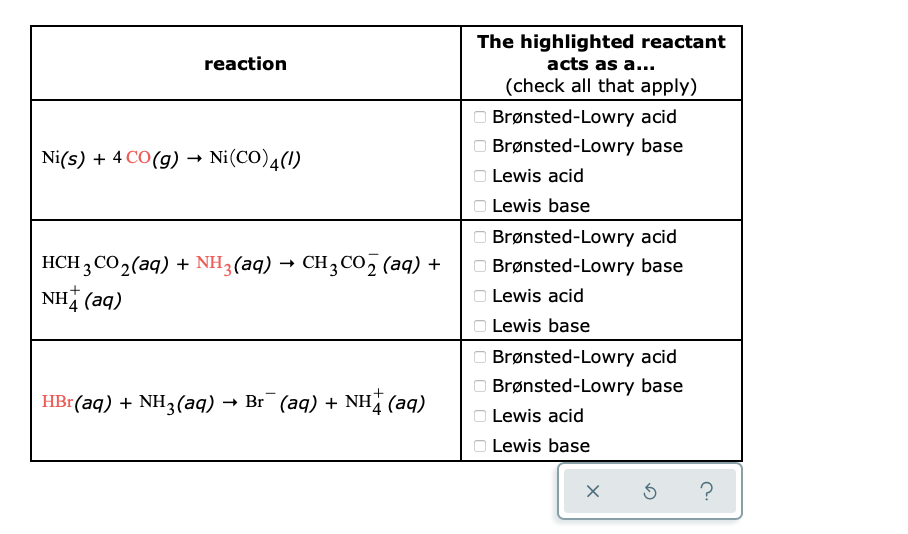
Identify the lewis acid and the lewis base in the following reactions. CaO+ CO2 to CaCO3 (ii) CH3-O-CH3+AlCl3 to
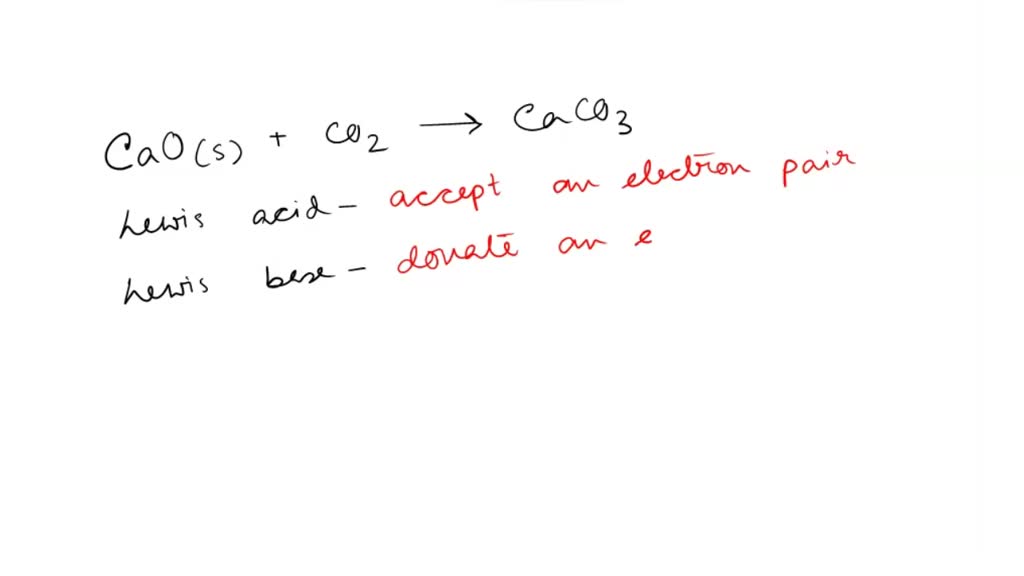
SOLVED: 'QUESTION 1 In the reaction: CaO(s) + C02(g) 5 CaCO3(s) 0A Ca2+acts as a Lewis acid and CO32- acts as a Lewis base 02-acts as a Lewis base and CO2 acts

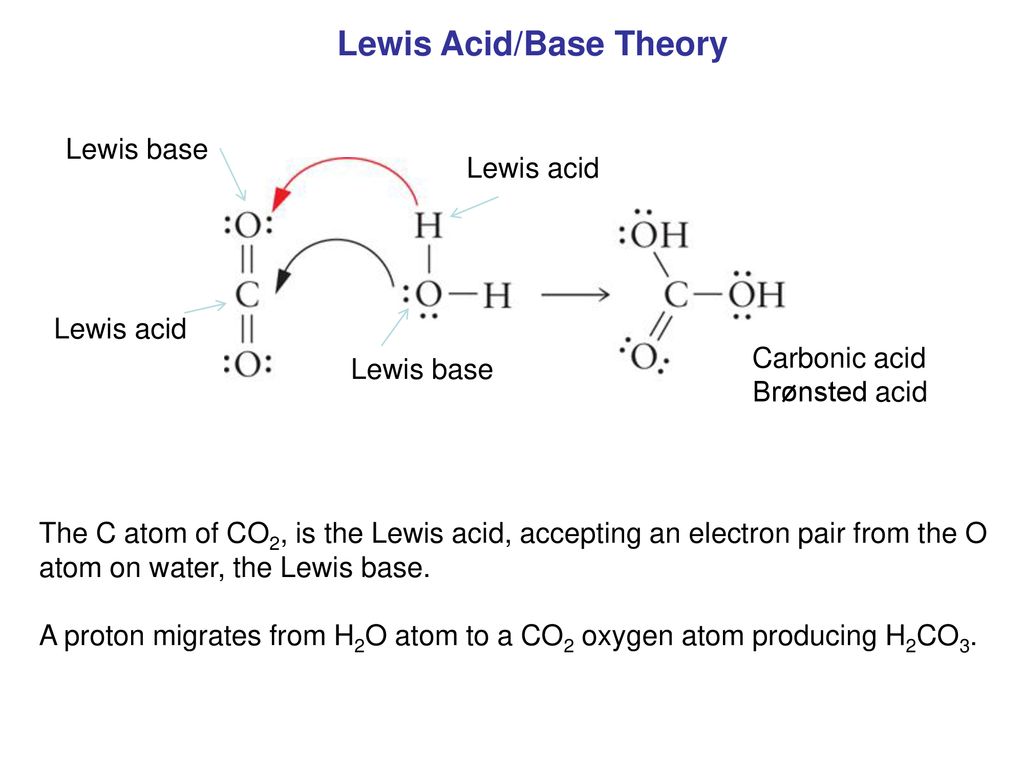
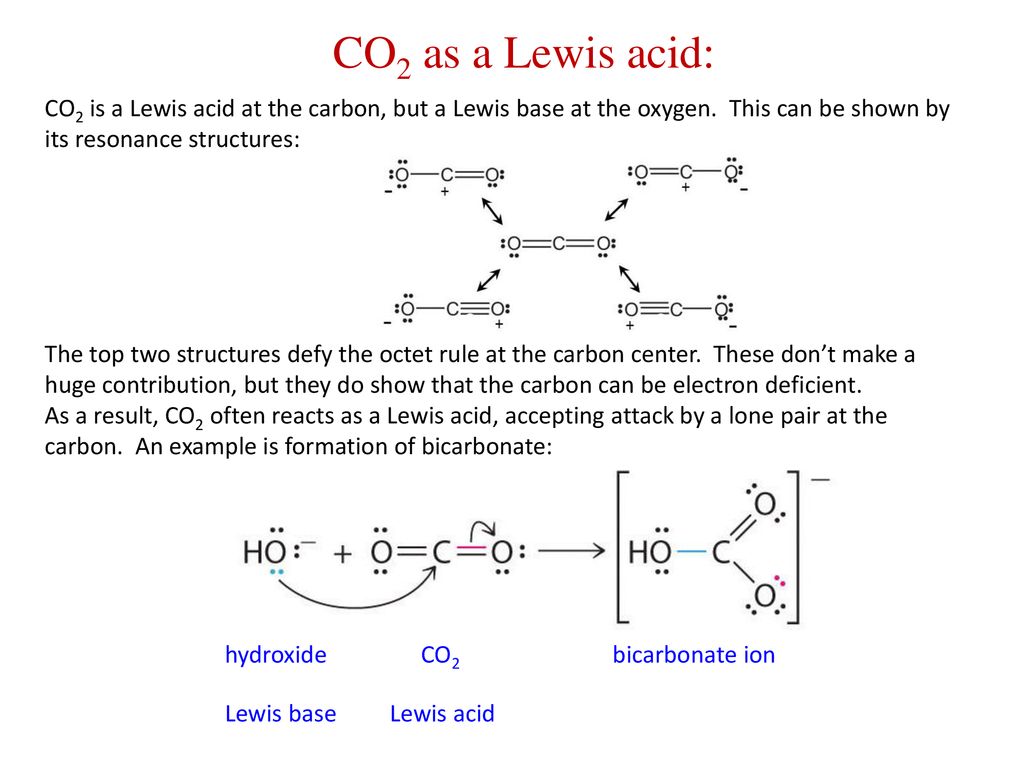
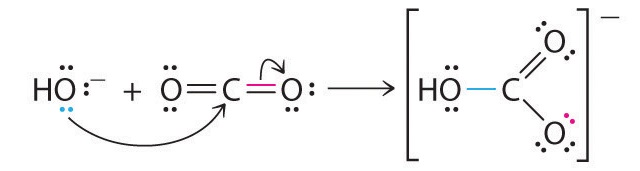
Role of the Surface Lewis Acid and Base Sites in the Adsorption of CO2 on Titania Nanotubes and Platinized Titania Nanotubes: An in Situ FT-IR Study | The Journal of Physical Chemistry

Porous metal-organic framework with Lewis acid−base bifunctional sites for high efficient CO2 adsorption and catalytic conversion to cyclic carbonates - ScienceDirect
![SOLVED: [ Which of the statements regarding the Lewis acid-base theory is FALSE? ] 1. All Bronsted Lowry bases are also Lewis bases. 2. Non-metal oxides such as CO2 and SO3 are SOLVED: [ Which of the statements regarding the Lewis acid-base theory is FALSE? ] 1. All Bronsted Lowry bases are also Lewis bases. 2. Non-metal oxides such as CO2 and SO3 are](https://cdn.numerade.com/previews/2f9ab192-509b-4230-b66b-21c281c3582f_large.jpg)